Guest post by Carlota Vergas
European Patent Attorney at Balder IP
Patent laws in Latin American countries
The Patent Laws in Latin American countries establish the common requirements for considering an invention to be patentable. Therefore, for an invention to be patentable it shall be industrially applicable, novel, and non-obvious, that is to say, the invention shall have an inventive step. Concerning the latter, most national patent offices in Latin America assess the inventive step requirement using, to an extent, the same analysis that the European Patent Office uses for deciding whether an invention is the result of an inventive step or not; this analysis is the so-called Problem and Solution approach, namely P&S approach.
How do patent offices in Latin America differ from the European Patent Office (EPO)?
Unlike the European Patent Office, many patent offices in Latin America do not apply the P&S approach in a strict manner even if the Guidelines or Manual for the Examination of Patent Applications thereof set out how the P&S approach is to be applied. In this respect, the national patent offices of countries of the Andean Community, which currently are Bolivia, Colombia, Ecuador and Peru, share a same Manual for the Examination of Patent Application (henceforth the Manual).
What is the Manual for Examination of Patent Application?
The Manual establishes that whenever possible, the P&S approach should be used. Briefly, in the P&S approach it is determined which is the piece of prior art that is closest to the claimed invention, the distinguishing features between the claimed invention and the prior art are identified, and the technical problem(s) that the distinguishing features solve is set out. Then, from the perspective of the person having ordinary skill in the art of the invention, it is determined whether it could and would have been obvious to modify the prior art to arrive at the claimed invention based on both the teachings of the prior art and the technical problem(s) to be solved.
In many occasions, Examiners of the patent offices of the Andean Community incorrectly identify the distinguishing features, and/or the objective technical problem(s) solved by the distinguishing features, and/or combine prior art documents in a rather artificial way because the person having ordinary skill in the art would not have been prompted to read the documents, or if read for some reason, she/he would not have combined them. So, it is not just whether the skilled person could combine the teachings, but whether she/he would have done so, the latter being frequently overlooked by Examiners. The skilled person thus must find a technical effect or motivation described in at least one of the prior art documents for her/him to combine the documents; also, the teachings in said documents must be compatible for the combination to be ‘obvious’.
Chemical example
For example, in relation to the particular case of compounds defined by a Markush formula, the Manual states that the existence of an effect or surprising use is the most common manner to establish inventiveness for new compounds, particularly when they have very similar structures. A Markush formula, e.g.,
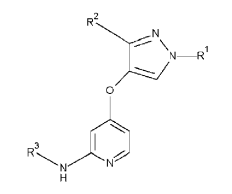
is used in patents to define a group of chemical compounds that share a common function and skeleton and differ in the meaning of the substituents, R1, R2 and R3 , in the present case, and can, for example, be used for the treatment of a certain disease “Y”.
The above referred surprising use is further described in the Manual as a completely different use from the known use (e.g. the treatment of a different disease “E”) or a substantial enhancement of a known effect (e.g. better compounds for treating the same disease “Y”).
In certain cases, and thus in line with the Manual, Examiners of the patent offices of the Andean Community request the presence of an improved technical effect associated with the new compounds; however, if the new compounds are “mere alternative” compounds to those already existing, (e.g. compounds that they are also useful for treating “Y”), convincing the Examiners that the new compounds are inventive is difficult, even with solid, persuasive arguments. Notwithstanding, it can be argued that the new compounds provide an effect, as established in the Manual, which is the same as that of the known compounds, for example, they are also effective for the treatment of disease Y. It can be further argued that alternatives are also inventions which enrich the state of the art, and the question to be answered in the analysis of inventive step according to the P&S approach is whether the alternative compounds are obvious or not in light of the state of the art.
Examiners often consider, in these cases, that replacing a substituent R1 in a known chemical structure by R4, for example an amino group (-NH2) by a hydroxyl (-OH) is within the skill of the expert, and conclude that there is no inventive step. When this occurs, an argument that can be put forward is that the examiner shall not consider whether the skilled person could have just made the changes in the chemical structure, but whether she/he would have done so with a reasonable expectation of success in providing also useful compounds for the treatment of disease “Y”. When Examiners assess whether the claimed invention is inventive, they shall take into account the teachings of the state of the art, the presence of pointers to the concrete replacements, and also the fact that minor changes in the structure of know compounds might have totally unexpected effects, not to mention that such effects cannot be predicted at all. Usually, when all these factors are taken into account, inventive step may be acknowledged.


